An Intramolecular Version of the Claisen Ester Condensation. Refer to the reaction below to answer the following questions.
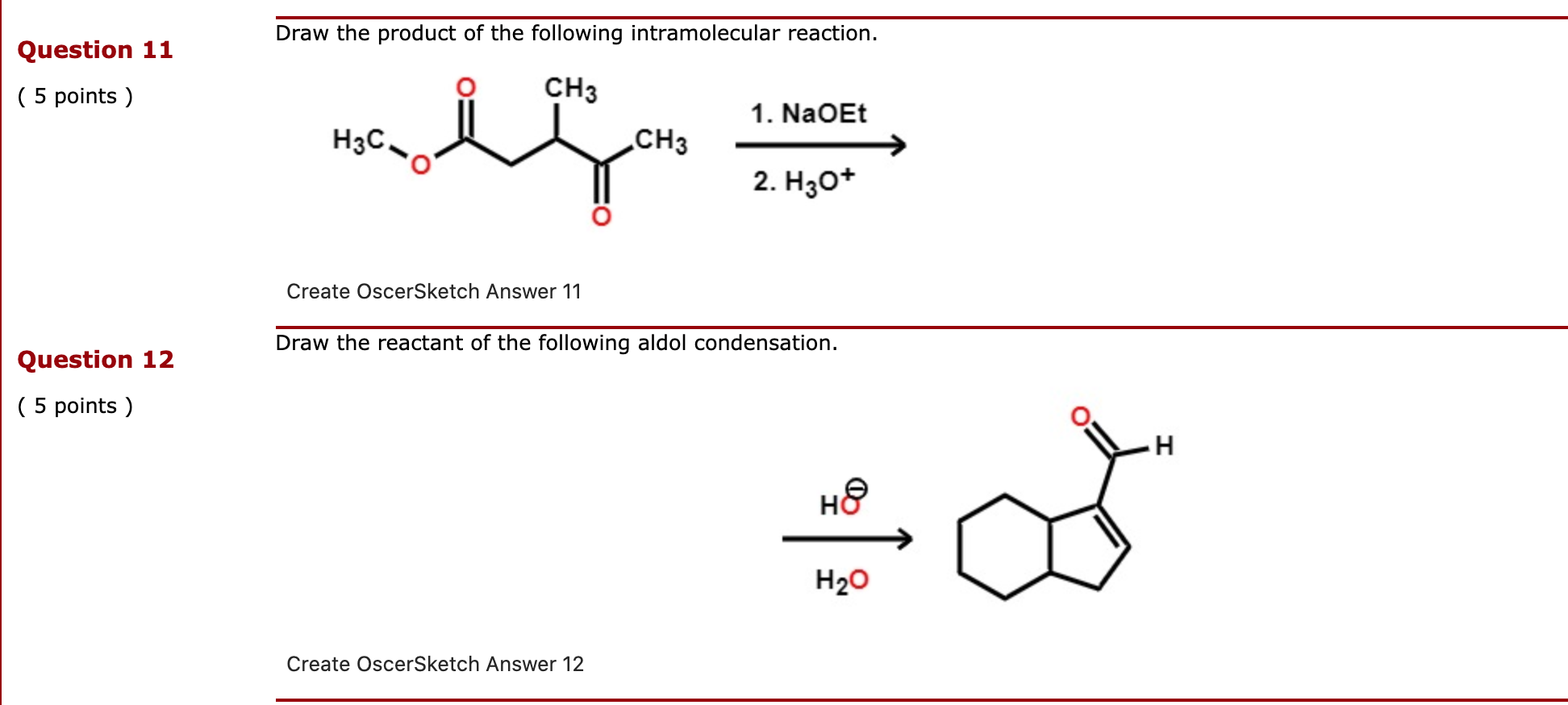
Solved Draw The Product Of The Following Intramolecular Chegg Com
Academiaedu is a platform for academics to share research papers.

. Like in the elimination reaction below for instance we get 80 of the tetrasubstituted alkene Zaitsev more substituted because there are 4 carbons attached to the alkene and 20 of the disubstituted non-Zaitsev product. Jan 20 2022 Draw the product that forms when the following product is treated with 1. The dehydration of aldol products to synthesize α β unsaturated carbonyls enones The products of aldol reactions often undergo a subsequent elimination of water made up of an alpha-hydrogen and the beta-hydroxyl group.
The p electrons of the aromatic CC act as a nucleophile attacking the electrophilic C. When a difunctional ester is used if the ester groups are positioned so that there are 4 or 5 carbon atoms between them an intramolecular version of the Claisen condensation can occur and is especially useful in the construction of five and six-membered rings. 12-addition reactions are all of those where the nucleophile attacks the carbonyl groupFor example the reaction of carbonyl compounds with.
So the Michael reaction is a particular type of conjugate addition reaction that ɑ β-unsaturated carbonyl compounds undergo with nucleophiles. Most of the time elimination reactions favor the more substituted alkene that is the Zaitsev product. Tert-butoxide can be used to form the less substituted alkenes in elimination reactions the E2 specifically.
However today well talk about one interesting exception to this rule and how under certain conditions we actually end up with the. Question 29 3 points Provide the major organic product in the reaction shown below. Which of the following compounds would you expect to be the major organic product of the two-step sequence shown here.
CI Draw the mechanism and product for the carbocation-mediated addition of 1 The stereospecificity of Claisen rearrangement arises from the symmetry-allowed suprasupra interaction between two 3-atom. The product of this beta-elimination reaction is an αβ-unsaturated aldehyde or ketone. As A Base tert-Butoxide Tends To Favor The Non-Zaitsev of Hofmann Product In Elimination Reactions.
Draw the major organic products. In general ɑ β-unsaturated carbonyl compounds can undergo a 12- or 14-addition reaction.

Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
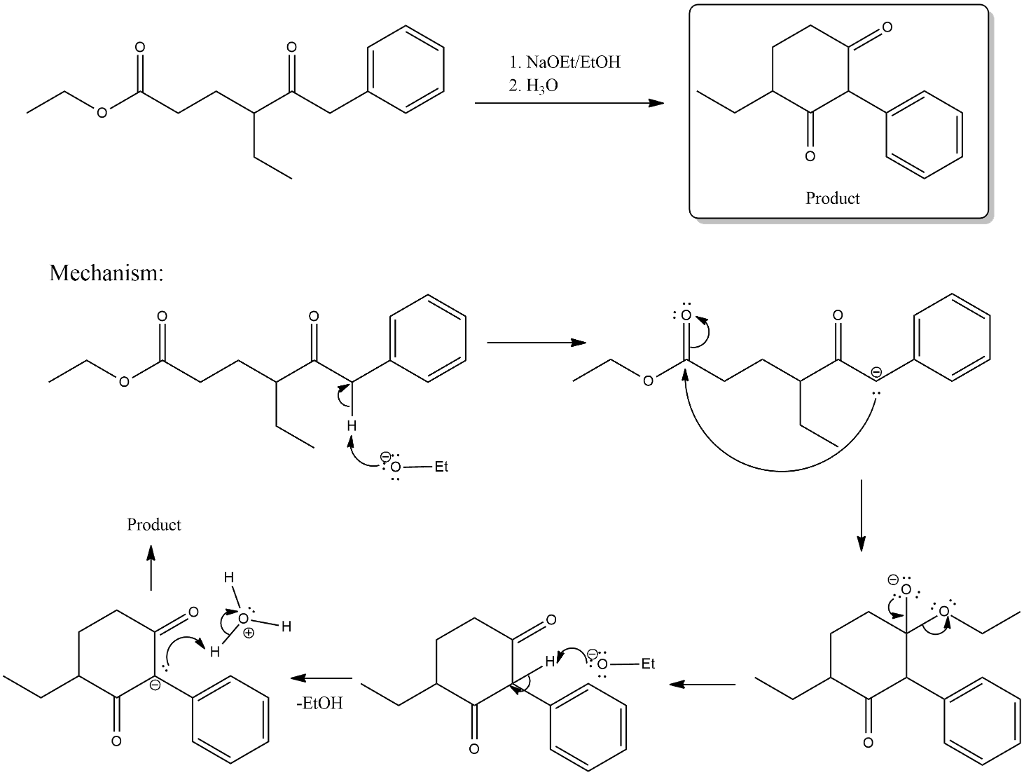
Oneclass Draw The Product Of The Below Intramolecular Claisen Condensation Draw Only The Major Tran
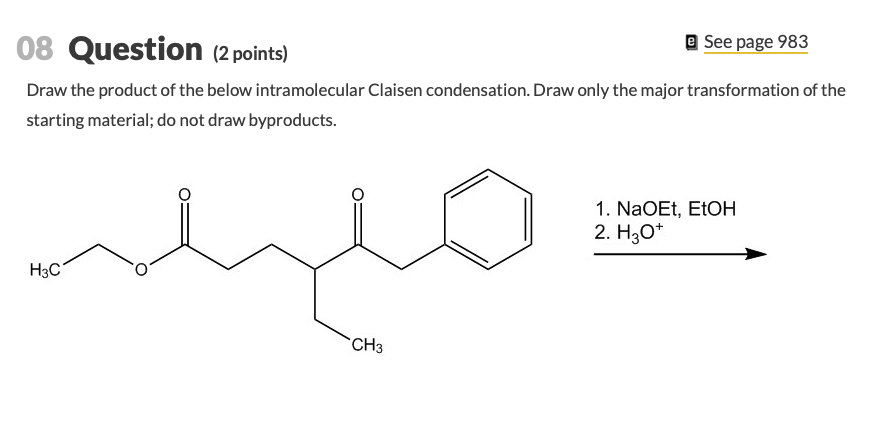
Solved 08 Question 2 Points E See Page 983 Draw The Chegg Com
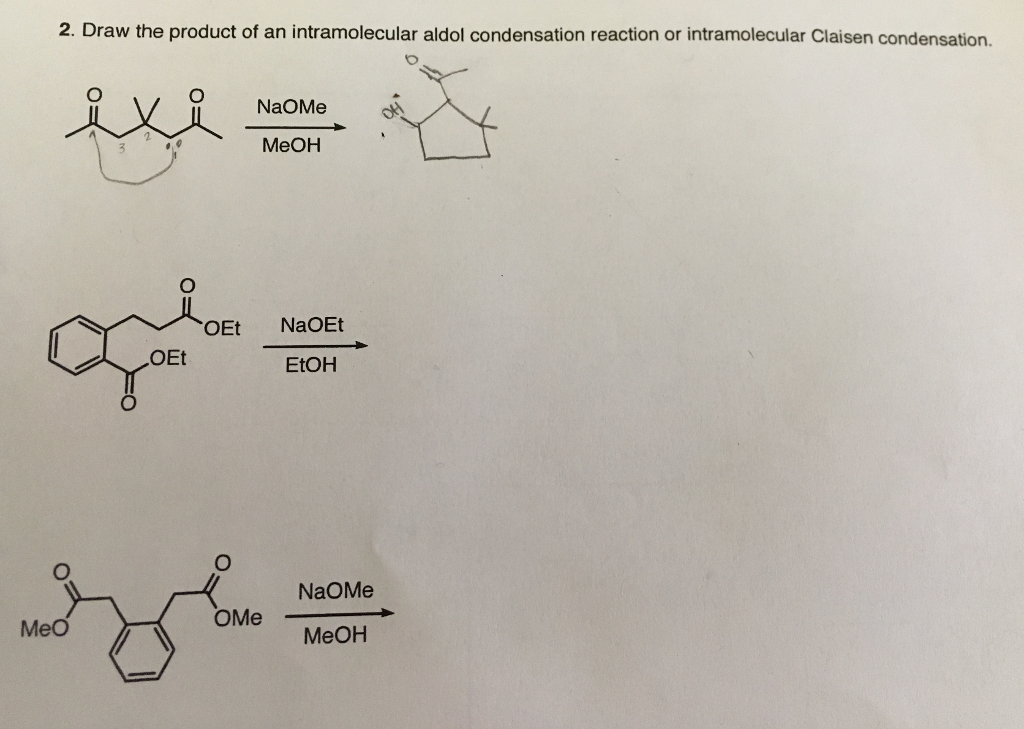
Solved 2 Draw The Product Of An Intramolecular Aldol Chegg Com
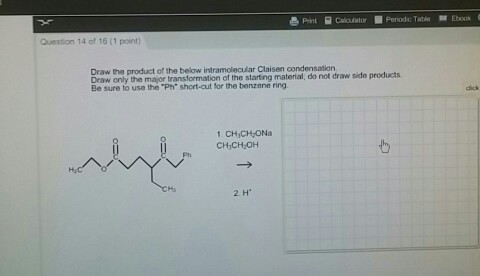
Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
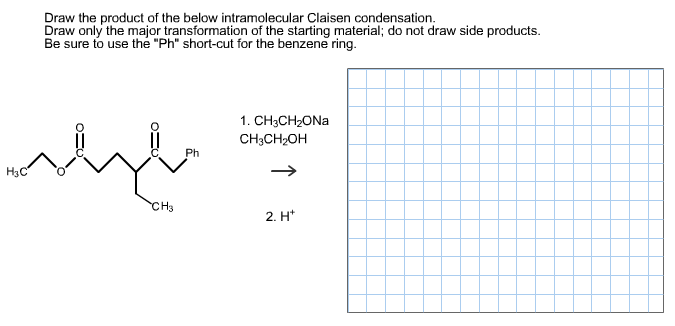
Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com

Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
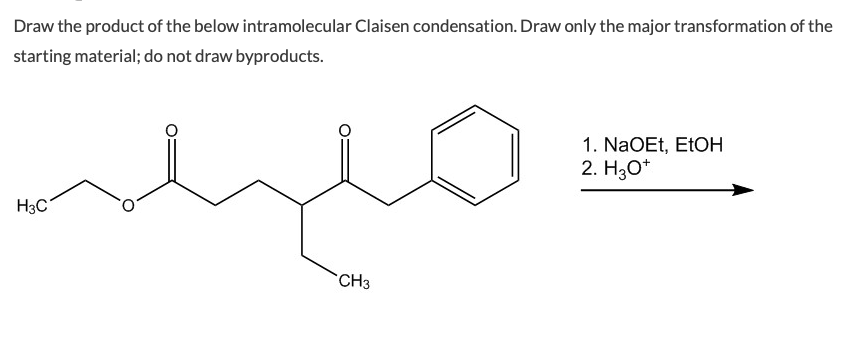
Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
0 comments
Post a Comment